Recently, the team of researchers Li Xianfeng and Zhang Huamin of the Energy Storage Technology Research Department (DNL17) of Dalian Institute of Chemical Physics, Chinese Academy of Sciences developed a manganese-based positive electrode pair based on two-electron transfer, deposition-dissolution reaction In the neutral zinc-manganese flow battery, the reliability of the battery is greatly improved. This work provides new ideas for the development of a new generation of secondary manganese-based batteries. Manganese-based batteries have received widespread attention due to their rich resource reserves, low cost, and environmental friendliness. However, problems such as poor cycle stability have been restricting the development of manganese-based batteries. The manganese-based pair proposed by the team uses Mn (Ac) 2 as the active material. Due to the coordination effect of Ac-, during the charging process, Mn (Ac) 2 is deposited on the electrode in the form of MnO2 through oxidation reaction. It dissolves into Mn (Ac) 2 during the process; it is completely different from other manganese salts (MnSO4 or MnCl2) in part or all to generate Mn3 +. This reaction can completely avoid the disproportionate side reaction of Mn3 +, thus greatly improving the stability of the electric pair And reversibility. The fundamental reason for the realization of the above MnO2 / Mn2 + reaction is that Mn (Ac) 2 is accompanied by the formation of HAc during the generation of MnO2, and the Gibbs free energy of HAc is much lower than that of free H + (MnSO4 or MnCl2 generates H + during the electrochemical reaction) , Thereby reducing the potential of the reaction as a whole and avoiding the occurrence of the Mn2 + / Mn3 + reaction. In addition, compared with the common zinc ion battery insertion / extraction mechanism, the dissolution / deposition reaction effectively avoids the phase change and structural collapse of the positive electrode during charging and discharging, thereby greatly improving the stability of the positive electrode. The test results of the assembled neutral zinc-manganese flow battery show that MnO2 can be uniformly deposited on the carbon fiber of the positive electrode, and its area capacity can reach 20mAh / cm2, which is the maximum value reported so far, and MnO2 can be completely discharged during the discharge process. Dissolved, the reversibility of the electrochemical reaction is very high. Compared with the traditional alkaline zinc-manganese system, the neutral electrolyte system is not corrosive, and can effectively avoid the problem of negative electrode zinc dendrites. Under the current density of 40mA / cm2, the zinc-manganese flow battery can run stably for more than 400 cycles, and the Coulomb efficiency can reach 99% and the energy efficiency can reach 78%. The related research results were published in "Energy and Environmental Science". The above work was supported by the National Natural Science Foundation of China and the STS project of the Chinese Academy of Sciences. Optical Crystals Aspherical Lens
China Star Optics has strong and comprehensive technical capability, integrate the design and manufacture as a whole.Imported the global advanced aspherical equipment and instruments,manufactured various aspherical products,including infrared material Zinc Selenide (ZnSe),Germanium (Ge),Silicon (Si),Zinc Sulfide (ZnS) etc. Aspheric lens has already application in various high technology project and products.
Aspheric Lens
Specification of our Aspherical Lens as follow: Optical Crystals Aspherical Lens,Optical Aspheric Lens,Ir Glass Aspheric Lens,Infrared Glass Aspherics Lens China Star Optics Technology Co.,Ltd. , https://www.realpoooptics.com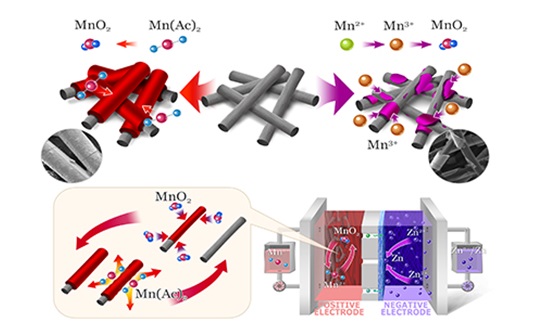
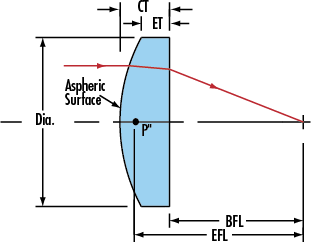
Material:BK7,B270 glass,PYREX,ZF13 or other optical infrared material Zinc Selenide (ZnSe),Germanium (Ge),Silicon (Si),Zinc Sulfide (ZnS) etc.
Size:10mm-200mm
Fringes:lambda/2
Center Thickness:15nm-25nm
Dispersion round:0.03mm-0.2mm
Centration:3 arc min
Surface Quality:60-40 Scratch/Dig
Clear Aperture:90%
Coating:Available upon Request
Dalian Chemical Research Institute developed a reversible two-electron dissolution-deposition type based on Mn2 + / MnO2
