Electrocatalytic decomposition of water to hydrogen is an important way to reduce environmental pollution and achieve renewable clean energy. It is of great scientific value and practical significance to develop an efficient and stable catalyst for hydrogen production. Graphene materials are widely used in the electrocatalytic decomposition of water for hydrogen because of its advantages of large specific surface area, good electrical conductivity and high stability. But so far, graphene materials have only been used as carriers for catalysts, and their hydrogen evolution capability has been enhanced through the use of cocatalyst loading or heteroatom doping. The related reports that graphene itself as a catalyst for electrocatalytic hydrogen evolution are relatively absent.
Researchers at the Key Laboratory of Organic Solids Institute of the Institute of Chemistry, Chinese Academy of Sciences have long been dedicated to chemical vapor deposition and etching of graphene, and have prepared a variety of graphenes with different layers, structures, dimensions and morphologies. Materials, effective control of the various properties of graphene. Recently, the research group cooperated with Wu Tianzhu Group, Key Laboratory of Photochemical Conversion and Functional Materials Institute, Institute of Physics and Chemistry, Chinese Academy of Sciences, and Ma Tianbao, Associate Professor of the State Key Laboratory of Tribology, Department of Mechanical Engineering, Tsinghua University, and worked directly under conditions without metal catalysts and templates. Three-dimensional graphene material was prepared, and the morphology of graphene was further regulated.
The three-dimensional graphene obtained from this research is composed of a high-density upright graphene-coated silica nanowire network, in which the silicon oxide nanowire network is prepared in-situ on a silicon substrate by chemical vapor deposition. Unlike inert two-dimensional planar graphenes, the high-density boundary sites in three-dimensional graphene materials can act as active centers for catalytic proton adsorption and reduction to produce hydrogen. This is the first time in the world that the shape of the graphene material has been controlled to realize high-efficiency electrocatalytic decomposition of water in the undoped and unsupported cocatalyst. Electrochemical measurements show that the initial voltage of the electrochemical hydrogen evolution of the three-dimensional graphene material optimized for morphology is only ~18 mV, which is very close to the commercial Pt/C. Theoretical studies have demonstrated that the excellent hydrogen evolution energy source is at the rich tip sites of three-dimensional graphene materials. These high-density boundary tip sites achieve the transition from catalytically inert intrinsic graphene to high catalytic performance. This study provides a theoretical and applied basis for the application of graphene materials in the field of hydrogen evolution in electrolysis.
Related research results were published in Angew. Chem. Int. Ed. The study was funded by the National Natural Science Foundation of China, the Ministry of Science and Technology, and the Chinese Academy of Sciences.
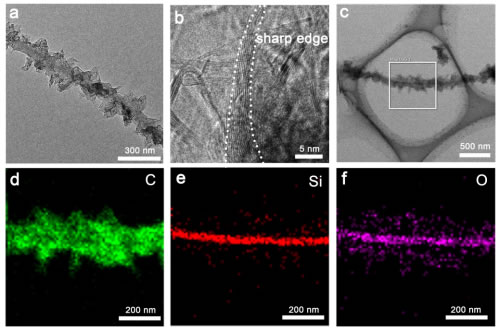
Figure 1. Three-dimensional graphene network with high density boundary sites
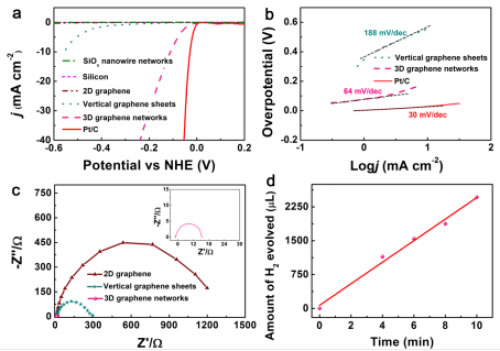
Figure 2. Electrocatalytic decomposition of three-dimensional graphene network materials
